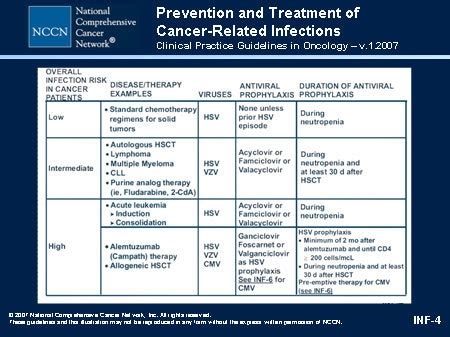
Are Antiviral Prophylaxis Guidelines the Same for Different Viruses?
Posted by Rick Ashworth, reviewed by Dr. Miguel Sanchez | 2024-Mar-15
The management of viral infections has been a longstanding challenge in the medical field, with healthcare professionals continually seeking to optimize preventive strategies. One critical aspect of this pursuit is the use of antiviral prophylaxis, which aims to reduce the risk of infection or mitigate the severity of symptoms. However, as the various viral pathogens exhibit distinct characteristics, it is essential to understand whether the guidelines for antiviral prophylaxis are universal or tailored to the specific virus in question.
Influenza Virus Prophylaxis
The influenza virus is a prime example of a pathogen where antiviral prophylaxis has been extensively studied and implemented. The Centers for Disease Control and Prevention (CDC) and other leading health organizations have developed comprehensive guidelines for the use of antiviral medications, such as oseltamivir and zanamivir, in both pre-exposure and post-exposure settings. These guidelines take into account factors like the individual's risk of developing severe illness, the timing of exposure, and the local epidemiological patterns of influenza strains.
HIV Prophylaxis
In the case of human immunodeficiency virus (HIV), the approach to antiviral prophylaxis is quite different. The World Health Organization (WHO) and other authorities have recommended the use of pre-exposure prophylaxis (PrEP) for individuals at high risk of HIV infection, such as those in serodiscordant relationships or those engaging in high-risk sexual behaviors. PrEP involves the regular use of antiretroviral medications, like tenofovir and emtricitabine, to prevent the acquisition of HIV. This strategy has proven effective in reducing the risk of HIV transmission and has been widely adopted in various settings.
COVID-19 Prophylaxis
The recent COVID-19 pandemic has shed light on the challenges of developing effective antiviral prophylaxis guidelines. While the CDC and other health organizations have provided recommendations for the use of vaccines and monoclonal antibodies in certain high-risk populations, the role of antiviral medications for prophylaxis remains a subject of ongoing research and debate. The constantly evolving nature of SARS-CoV-2, the causative agent of COVID-19, and the emergence of new variants have added complexity to the development of universal prophylactic strategies.
Implications for Clinical Practice
The variations in antiviral prophylaxis guidelines across different viral infections have significant implications for clinical practice. Healthcare providers must stay up-to-date with the latest recommendations and tailor their approach accordingly. This requires a comprehensive understanding of the unique characteristics of each virus, the available prophylactic options, and the potential benefits and risks associated with their use.
Moreover, the implementation of effective antiviral prophylaxis strategies often relies on factors beyond the clinical setting, such as public health policies, population-level vaccination coverage, and access to healthcare resources. Addressing these broader aspects is crucial for ensuring the optimal use of antiviral prophylaxis and reducing the burden of viral infections on individuals and communities.
As the field of virology continues to evolve, healthcare professionals must remain vigilant and adaptable in their approach to antiviral prophylaxis. By staying informed and continuously evaluating the guidelines for different viral pathogens, clinicians can make informed decisions that optimize patient outcomes and contribute to the overall public health response.
What are your thoughts on the variations in antiviral prophylaxis guidelines across different viral infections? Share your insights and experiences in the comments below.