How do breakpoints influence the interpretation of antimicrobial susceptibility testing results?
Explain the concept of breakpoints in antimicrobial susceptibility testing and their significance in categorizing bacterial isolates as susceptible, intermediate, or resistant to specific antibiotics. Discuss the role of breakpoints in guiding clinical decision-making regarding antibiotic therapy and treatment outcomes.
The Significance of Breakpoints in Antimicrobial Susceptibility Testing
Posted by Rick Ashworth, reviewed by Dr. Miguel Sanchez | 2024-Apr-02
Antimicrobial susceptibility testing (AST) is a crucial diagnostic tool used to determine the effectiveness of antibiotics against specific bacterial isolates. At the core of this testing process are breakpoints, which play a pivotal role in interpreting the results and guiding clinical decision-making. Understanding the concept of breakpoints and their influence on AST interpretations is essential for optimizing antibiotic therapy and improving treatment outcomes.
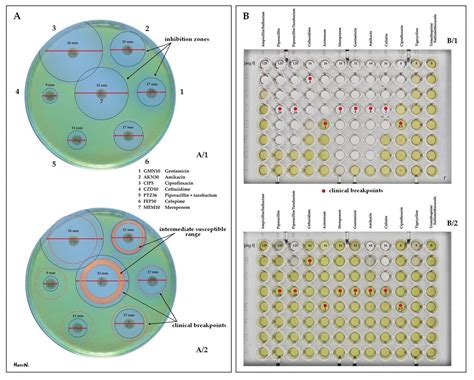
Breakpoints are defined as the specific minimum inhibitory concentration (MIC) or zone diameter values that categorize bacterial isolates as susceptible, intermediate, or resistant to a particular antibiotic. These thresholds are established by regulatory bodies, such as the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST), based on a comprehensive analysis of factors, including pharmacokinetic and pharmacodynamic data, clinical outcomes, and epidemiological cutoff values.
The significance of breakpoints lies in their ability to translate the numerical AST results into clinically meaningful interpretations. Susceptible organisms are those for which the antibiotic is likely to be effective, while resistant organisms are unlikely to respond to the antibiotic. The intermediate category represents a "grey zone" where the antibiotic may have limited efficacy, and alternative treatment options or dose adjustments may be necessary.
Breakpoints play a crucial role in guiding clinical decision-making regarding antibiotic therapy. By categorizing bacterial isolates, healthcare providers can make informed decisions about the most appropriate antimicrobial agent to prescribe, taking into account the patient's clinical condition, the severity of the infection, and the potential for adverse effects or drug interactions. This information is particularly important in the era of antimicrobial resistance, where the selection of the right antibiotic can significantly impact treatment outcomes and prevent the further spread of resistant strains.
It is important to note that breakpoints are not static and can be revised over time as new data becomes available. Factors such as changes in antibiotic usage patterns, the emergence of new resistance mechanisms, or advancements in pharmacokinetic and pharmacodynamic knowledge may prompt regulatory bodies to update breakpoint values. Healthcare providers must stay abreast of these changes to ensure that their interpretation of AST results remains accurate and aligned with the latest clinical guidelines.
Moreover, the interpretation of AST results can be influenced by various factors beyond the established breakpoints. These may include the specific testing method used, the quality of the sample, the presence of synergistic or antagonistic interactions between antibiotics, and the patient's individual characteristics, such as immune status and comorbidities.
In conclusion, breakpoints are essential in the interpretation of antimicrobial susceptibility testing results, serving as a critical link between the laboratory data and clinical decision-making. By understanding the significance of breakpoints and their dynamic nature, healthcare providers can optimize antibiotic therapy, improve treatment outcomes, and contribute to the global effort in combating antimicrobial resistance. As the field of infectious disease continues to evolve, the role of breakpoints in guiding clinical practice will remain crucial for ensuring the effective and responsible use of these vital medical tools.
User comments
More Topics to Explore
How does antibiotic resistance affect antimicrobial susceptibility testing?
Discuss the impact of antibiotic resistance on the accuracy of antimicrobial susceptibility testing and the challenges it presents in treating bacterial infections. Share your insights and experiences on this important issue.
What are the key technologies used in antimicrobial susceptibility testing?
Delve into the various technologies such as automated systems, disk diffusion, and gradient diffusion used in antimicrobial susceptibility testing. Share the advantages, limitations, and future prospects of these technologies in predicting effective antibiotic treatments.
How can healthcare professionals improve the accuracy of antimicrobial susceptibility testing results?
Share best practices, strategies, and tips for enhancing the precision and reliability of antimicrobial susceptibility testing outcomes. Discuss the role of proper specimen collection, laboratory techniques, and interpretation of results in optimizing patient care and antibiotic therapy.
What are the emerging trends in antimicrobial susceptibility testing methods?
Explore the latest developments and advancements in antimicrobial susceptibility testing, including genotypic methods, MALDI-TOF mass spectrometry, and rapid molecular techniques. Discuss the potential impact of these novel approaches on enhancing the speed and accuracy of identifying antibiotic-resistant pathogens.
Is there a correlation between biofilm formation and antimicrobial susceptibility testing?
Examine the relationship between biofilm-producing bacteria and their resistance to antimicrobial agents tested in standard susceptibility assays. Discuss the challenges posed by biofilm-associated infections in clinical settings and strategies to address biofilm-mediated antibiotic resistance.
What role does antimicrobial stewardship play in enhancing antimicrobial susceptibility testing?
Discuss the importance of antimicrobial stewardship programs in promoting rational antibiotic use, preserving efficacy, and combating resistance. Share insights on how antimicrobial stewardship initiatives can support accurate antimicrobial susceptibility testing and optimal antibiotic prescribing practices.
What are the challenges in conducting antimicrobial susceptibility testing for atypical pathogens?
Explore the unique challenges and considerations involved in performing susceptibility testing for atypical pathogens like Mycoplasma, Chlamydia, and Legionella. Discuss the limitations of standard testing methods and the importance of tailored approaches to accurately assess antimicrobial susceptibility in these cases.
How can advances in data analytics enhance the accuracy of antimicrobial susceptibility testing?
Explore the potential applications of data analytics, artificial intelligence, and machine learning in improving the precision and efficiency of antimicrobial susceptibility testing. Discuss how predictive modeling and big data analysis can optimize antibiotic selection, dosing regimens, and patient outcomes based on susceptibility test results.
What are the ethical considerations in antimicrobial susceptibility testing and reporting of results?
Reflect on the ethical dilemmas surrounding antimicrobial susceptibility testing, including issues of result interpretation, reporting, confidentiality, and patient autonomy. Discuss the principles of responsible antibiotic stewardship, patient consent, and equitable access to effective treatments in the context of antimicrobial susceptibility testing practices.