The Genetic Influence on Antiviral Drug Pharmacokinetics
Posted by Rick Ashworth, reviewed by Dr. Miguel Sanchez | 2024-Apr-09
As the world continues to battle a variety of viral infections, the development and optimization of antiviral drugs have become increasingly crucial. However, the effectiveness of these medications can be heavily influenced by an often overlooked factor - genetic variations within the patient population. Exploring the intricate relationship between an individual's genetic makeup and the pharmacokinetics of antiviral drugs is a critical area of research that holds the potential to revolutionize personalized treatment approaches.
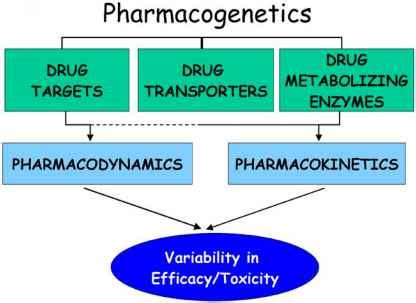
At the heart of this complex interplay lies the role of pharmacogenomics, the study of how an individual's genetic profile affects their response to medications. When it comes to antiviral drugs, genetic variations can influence key processes such as drug absorption, distribution, metabolism, and elimination (ADME). These factors ultimately determine the concentration of the drug within the body and, consequently, its therapeutic efficacy and potential for adverse reactions.
One of the primary ways in which genetics can impact antiviral drug pharmacokinetics is through the cytochrome P450 (CYP) enzyme system. This family of enzymes is responsible for the metabolism of a vast array of medications, including many antiviral agents. Genetic polymorphisms in the genes encoding these enzymes can lead to altered enzyme activity, resulting in variations in drug clearance rates and, ultimately, drug exposure within the body.
For example, the metabolism of the antiviral drug oseltamivir, commonly used to treat influenza, is primarily facilitated by the CYP2D6 enzyme. Individuals with genetic variants that result in reduced CYP2D6 activity may experience higher concentrations of the active metabolite of oseltamivir, potentially leading to an increased risk of adverse effects. Conversely, those with genetic variants associated with enhanced CYP2D6 activity may clear the drug more rapidly, potentially compromising its therapeutic efficacy.
Another crucial factor is the impact of genetics on drug transporters, which are responsible for the movement of drugs across cellular membranes. Variations in the genes encoding these transporters can affect the distribution and accumulation of antiviral medications within target tissues, ultimately influencing their pharmacological activity.
The human immunodeficiency virus (HIV) represents an area where the interplay between genetics and antiviral drug pharmacokinetics has been extensively studied. The effectiveness of antiretroviral therapy (ART) for HIV treatment is heavily dependent on the patient's genetic profile. Genetic variations in genes involved in drug metabolism and transport, such as CYP3A4, ABCB1, and SLCO1B1, have been associated with altered pharmacokinetics and treatment outcomes for various antiretroviral medications.
Understanding these genetic factors is crucial for optimizing antiviral drug therapy and minimizing the risk of treatment failure or adverse events. By incorporating pharmacogenomic testing into clinical practice, healthcare providers can tailor antiviral drug regimens to the individual patient, ensuring enhanced therapeutic efficacy and improved patient outcomes.
As the field of personalized medicine continues to evolve, the integration of genetic information into the management of viral infections holds immense promise. By unraveling the complex relationship between an individual's genetic makeup and the pharmacokinetics of antiviral drugs, we can pave the way for more precise and effective treatment strategies, ultimately improving the quality of life for patients battling viral diseases.
What other genetic factors do you believe could influence the pharmacokinetics of antiviral medications? Share your insights in the comments below.