Antiviral Resistance Testing in Influenza: A Critical Tool for Combating Evolving Threats
Posted by Rick Ashworth, reviewed by Dr. Miguel Sanchez | 2024-Mar-18
As the influenza virus continues to adapt and evolve, the need for effective antiviral strategies has become increasingly paramount. Antiviral resistance testing has emerged as a crucial component in the arsenal against this persistent public health challenge, offering invaluable insights that can guide clinicians in delivering tailored, evidence-based care.
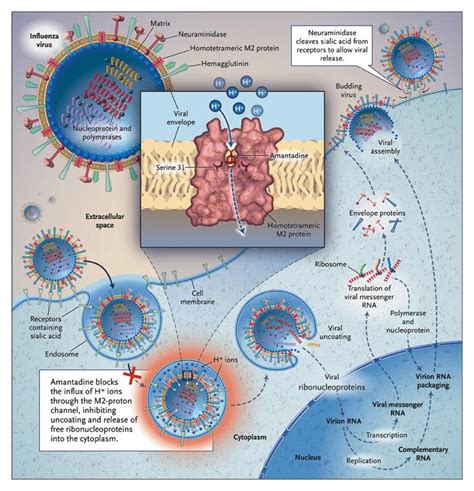
Influenza, a highly contagious respiratory illness, has long been a formidable foe, causing significant morbidity and mortality worldwide. The virus's remarkable ability to mutate and evade immune defenses has made it a moving target, with the potential to render previously effective treatments obsolete. This dynamic landscape underscores the importance of proactively monitoring for the emergence of antiviral-resistant strains.
Antiviral resistance testing plays a pivotal role in this endeavor, providing clinicians with a clear understanding of the susceptibility of the circulating influenza viruses to available antiviral medications. By conducting these specialized tests, healthcare providers can make informed decisions about the most appropriate course of treatment, ensuring that patients receive the most effective therapies and minimizing the risk of treatment failure.
Moreover, the data gathered from antiviral resistance testing can have far-reaching implications beyond individual patient care. By identifying the prevalence and distribution of resistant strains, public health authorities can better monitor the evolution of the influenza virus, enabling them to develop targeted intervention strategies and adapt vaccination programs accordingly. This collective understanding can lead to more effective containment efforts, ultimately safeguarding the broader population from the potential consequences of widespread antiviral resistance.
Implementing antiviral resistance testing in clinical practice, however, is not without its challenges. Ensuring timely access to these specialized tests, interpreting the complex results, and seamlessly integrating the findings into treatment decision-making can be daunting tasks for healthcare providers. Fortunately, guidelines and best practices have emerged to help navigate these complexities.
The Centers for Disease Control and Prevention (CDC) and other leading healthcare organizations have developed comprehensive recommendations for the use of antiviral resistance testing in influenza management. These guidelines emphasize the need for close collaboration between clinicians, public health authorities, and laboratory experts to establish robust surveillance networks and streamline the testing process. By adhering to these established protocols, healthcare providers can leverage the power of antiviral resistance testing to optimize patient outcomes and contribute to the larger effort to combat the evolving threat of influenza.
As we continue to navigate the unpredictable landscape of influenza, the role of antiviral resistance testing will only become more critical. By embracing this valuable tool and incorporating it into our clinical practice, we can empower ourselves to make more informed decisions, tailor treatment strategies, and ultimately, enhance our ability to protect individuals and communities from the ravages of this resilient virus.